
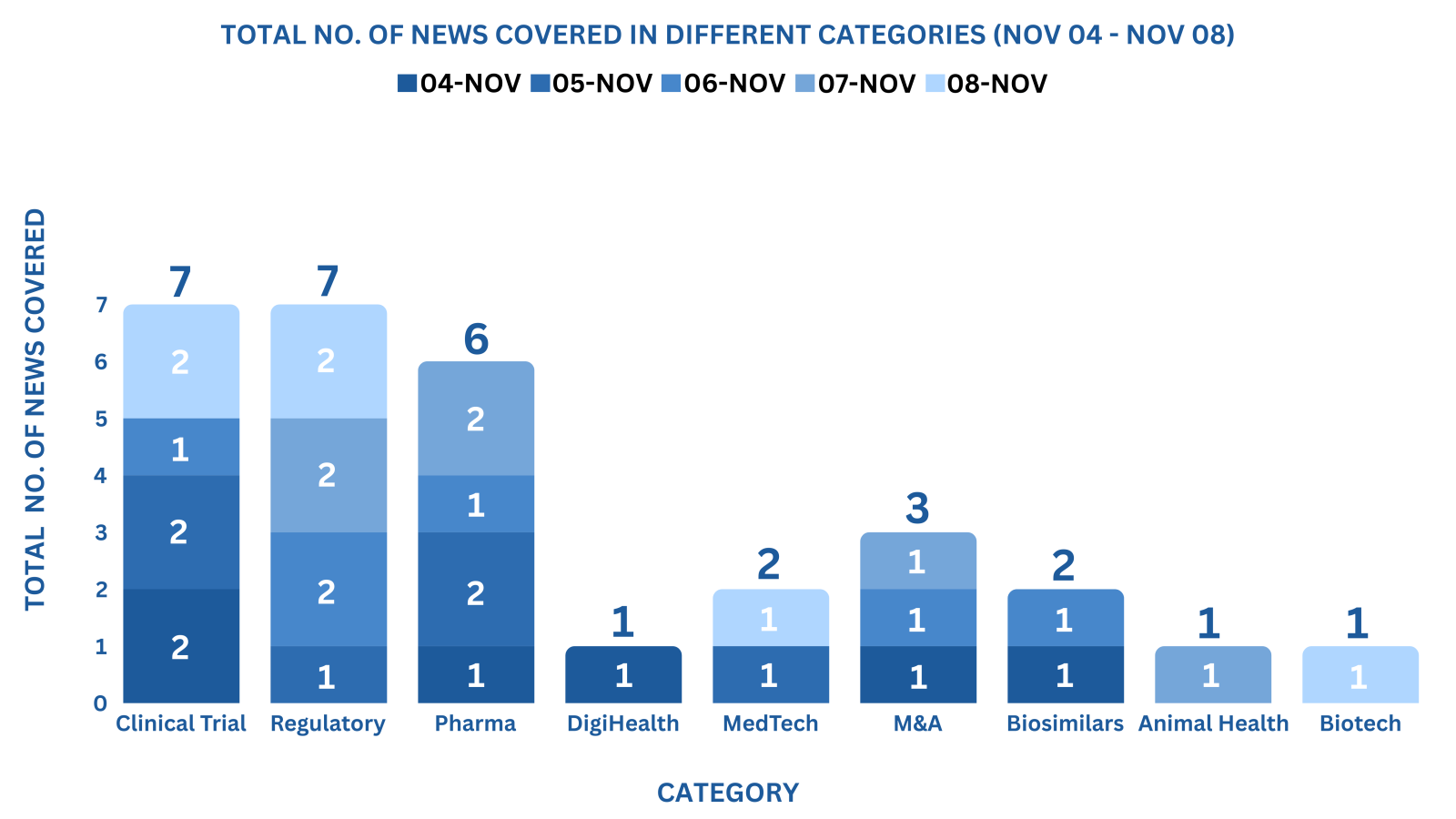
PharmaShots Weekly Snapshots (November 04 – November 08, 2024)
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, DigiHealth, MedTech, M&A, Biosimilar, Animal Health & Biotech. Check out our full report below:

Formosa Reports Top-Line Data from P-III (CPN-303) Study of APP13007 for Inflammation and Pain Post Cataract Surgery in Chinese Patients
Read More: Formosa Pharmaceuticals
Cytoki Pharma Reports the First Patient Dosing with CK-0045 in the P-II Trial for Treating Obesity and Type 2 Diabetes
Read More: Cytoki Pharma
Xencor Reports the First Individual Dosing with XmAb942 in P-I/II Trial to Treat Inflammatory Bowel Disease
Read More: Xencor
Zymeworks Reports the First Patient Dosing with ZW191 in P-I Study for Treating Folate Receptor-⍺ Expressing Advanced Solid Tumors
Read More: Zymeworks
Minghui Pharmaceutical Reports Initial Data from P-I Study of MHB039A for R/R Solid Tumors
Read More: Minghui Pharmaceutical
OncoResponse Reports P-I Study Results of OR502 for the Treatment of Advanced Cancer
Read More: OncoResponse
AstraZeneca Reports Data from the P-III (WAYPOINT) Study of Tezspire (Tezepelumab) for Chronic Rhinosinusitis with Nasal Polyps
Read More: AstraZeneca

Ionis Reports the US FDA’s NDA Acceptance of Donidalorsen as a Prophylactic Treatment of Hereditary Angioedema (HAE)
Read More: Ionis
Sanofi and Regeneron Reports the EC’s Approval of Dupixent (Dupilumab) for Young Children with Eosinophilic Esophagitis (EoE)
Read More: Sanofi and Regeneron
Leads Biolabs Secures the US FDA’s Orphan Drug Designation for LBL-034 to Treat Multiple Myeloma
Read More: Leads Biolabs
ARTHEx Biotech Secures the US FDA’s Rare Pediatric Designation (RPD) to ATX-01 for Treating Myotonic Dystrophy Type 1 (DM1)
Read More: ARTHEx Biotech
Cumberland Pharmaceuticals’ Ifetroban Secures the US FDA’s ODD and RPDD for Duchenne Muscular Dystrophy
Read More: Cumberland Pharmaceuticals
Dizal Reports the Regulatory Submission of Sunvozertinib to the US FDA for Treating R/R Non-Small Cell Lung Cancer
Read More: Dizal
AskBio’s AB-1003 Secures the US FDA’s Rare Pediatric Disease and Orphan Drug Designations to Treat Limb-Girdle Muscular Dystrophy Type 2I/R9
Read More: AskBio

AbbVie Join Forces with EvolveImmune Therapeutics for Developing Next-Generation Biotherapeutics in Oncology
Read More: AbbVie and EvolveImmune Therapeutics
Surrozen Collaborates with TCGFB for Developing TGF-β Antibodies for Idiopathic Pulmonary Fibrosis
Read More: Surrozen and TCGFB
Ascendis Pharma Join Forces with Novo Nordisk to Develop and Commercialize TransCon Tech-based Products in Metabolic and Cardiovascular Indications
Read More: Ascendis Pharma and Novo Nordisk
Inocras and Summit Pharmaceuticals International Corporation Join Forces to Advance Healthcare in Japan
Read More: Inocras and Summit Pharmaceuticals
Naitive Technologies Join Forces with Parvizi Surgical Innovation to Advance Osteoporosis Detection
Read More: Naitive Technologies and Parvizi Surgical Innovation
Aditum Bio and Leads Biolabs Launch Oblenio Bio to Advance LBL-051 for Autoimmune Indications
Read More: Aditum Bio and Leads Biolabs

Medable Collaborates with Google Cloud to Offer Digital Clinical Trials Technology on Google Cloud Marketplace
Read More: Medable and Google Cloud

Abbott Reports 2 Years Data from the LIFE-BTK Study of Esprit BTK System to Treat Peripheral Artery Disease Below the Knee
Read More: Abbott
Johnson & Johnson MedTech Reports the US FDA’s Approval of VARIPULSE Pulsed Field Ablation Platform to Treat Atrial Fibrillation
Read More: Johnson & Johnson

Jade Biosciences Reverse Merges with Aerovate Therapeutics to Advance Standard of Care for Autoimmune Diseases
Read More: Jade Biosciences and Aerovate Therapeutics
Boston Scientific Reports the Acquisition of Cortex
Read More: Boston Scientific and Cortex
GHO Capital Partners and Ampersand Capital Partners Report the Acquisition of Avid Bioservices for ~$1.1B
Read More: GHO Capital Partners, Ampersand Capital & Avid Bioservices

Meitheal Pharmaceuticals Receives Exclusive Commercial Rights of Three Biosimilars Across the US
Read More: Meitheal Pharmaceuticals
Alvotech and Advanz Pharma Reports the EMA’s MAA Acceptance of AVT05 (Biosimilar, Simponi)
Read More: Alvotech and Advanz Pharma

Jaguar Health Reports the US FDA’s Renewal of Conditional Approval for Canalevia-CA1 to Treat Chemotherapy-Induced Diarrhea in Dogs
Read More: Jaguar Health

Pheast Therapeutics Highlights Preclinical Data of PHST001 at SITC 2024
Read More: Pheast Therapeutics
Related Post: PharmaShots Weekly Snapshots (October 28 – October 30, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














